SureSet Announces Partnership with Business Asia Consultants to Provide International Marketing and Sales Services
The partnership will provide strategic marketing and positioning of the SureSet Critical Care line of products internationally.
We expect the agreement with BAC to accelerate our ability to expand the sales of our products into multiple international markets”
LOWELL, MA, UNITED STATES, February 2, 2022 /EINPresswire.com/ -- MedicaMetrix, Inc., a leader in developing and marketing innovative medical devices and healthcare solutions, has signed an agreement as the company begins its international distribution of the SureSet Securement line of products.— Perry Borch - SureSet Director of Sales & Business Development
Under the agreement, Business Asia Consultants (BAC) will provide a myriad of services including strategic market planning, program development and corporate positioning for SureSet in international markets. Additionally, BAC will utilize its expertise to oversee the Import/Export management as well as the Regulatory Strategy Pathway and Support.
“We expect the agreement with BAC to accelerate our ability to expand the sales of our products into multiple international markets” said Perry Borch, Director – Sales and Business Development for MedicaMetrix. “The expertise that Larry Kronick and his team bring to us will be indispensable as we plan our 2022 growth around the world.”
Business Asia Consultants is led by founder and CEO, Lawrence (Larry) Kronick. Mr. Kronick has over 35 years of sales and distribution management experience in Asia-Pacific, Latin America, North America, Middle East, and Europe. The company has international offices in seven locations including Hong Kong, Germany, China, and Brazil.
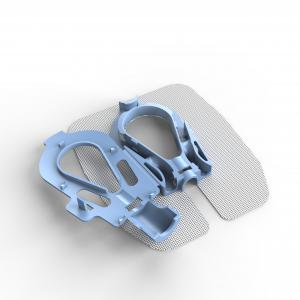
The MedicaMetrix Critical Care Division is currently focused on the sales and marketing of SureSet – an innovative peripheral IV securement device. The division is led by Perry Borch and is aiming to begin sales and distribution of SureSet devices in the first quarter of 2022.
About MedicaMetrix:
MedicaMetrix develops innovative technologies and device solutions that transform the healthcare status quo, leading to better medical outcomes, streamlined care and enhanced patient experience.
We are leading the development of a new paradigm that transforms the diagnosis, treatment, and management of prostate health by filling the gap between PSA testing and imaging / biopsies. The ProstaMetrix system is a minimally invasive medical device designed to accurately measure the volume of the prostate gland early in the diagnostic process. ProstaMetrix helps physicians assess a patient’s prostate status to better plan and monitor diagnostic procedures, treatments, drug therapies, and guide recommendations for active surveillance versus prostate biopsies.
MedicaMetrix is planning to acquire and develop other new medical devices with the goal to bring them to market rapidly by leveraging our planned production facilities in the U.S. and India.
Cautionary Statements Concerning Forward-Looking Statements:
This press release contains “forward-looking” statements as defined in the Private Securities Litigation Reform Act of 1995. These statements are based on management's current expectations or predictions of future conditions, events, or results based on various assumptions and management's estimates of trends and economic factors in the markets in which we are active, as well as our business plans. Words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “projects,” “forecasts,” “continue,” “may,” “should,” “will,” “goals,” and variations of such words and similar expressions are intended to identify such forward-looking statements. The forward-looking statements may include, without limitation, statements related to the expected impact of COVID-19 on our business, statements regarding our growth, product development, product potential, financial performance, sales growth, product adoption, market awareness of our products, data validation, our assessment of our internal controls over financial reporting, our visibility at and sponsorship of conferences and educational events. The forward-looking statements are and will be subject to risks and uncertainties, which may cause actual results to differ materially from those expressed or implied in such forward-looking statements. Forward-looking statements contained in this press release should be evaluated together with the many uncertainties that affect our business and our market, as well as other risks and cautionary statements set forth in our filings with the U.S. Securities and Exchange Commission. Forward-looking statements are not a guarantee of future performance, and actual results may differ materially from those projected. The forward-looking statements are representative only as of the date they are made and, except as required by applicable law, we assume no responsibility to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances, or otherwise.
MedicaMetrix, Inc.
InvestorRelations@medicametrix.com
Source: MedicaMetrix, Inc.
Released February 2, 2022
Jamie Jamitkowski
MedicaMetrix Inc.
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn